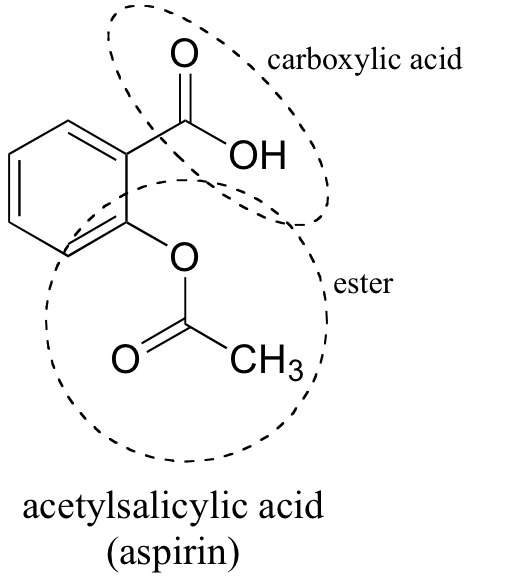
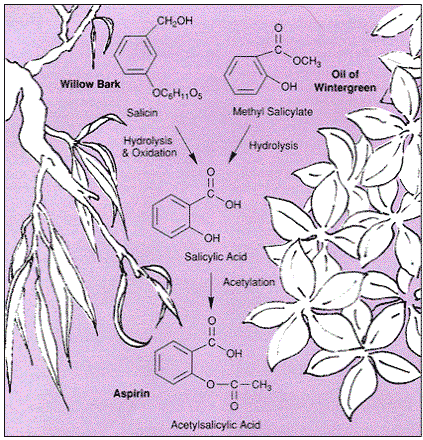
Structural representations of acetylsalicylic acid (1a) and salicylic... | Download Scientific Diagram

FeCl_3 reacts with only one of the following compounds : aspirin and salicylic acid. Which part of a molecule does FeCl_3 react ? | Homework.Study.com
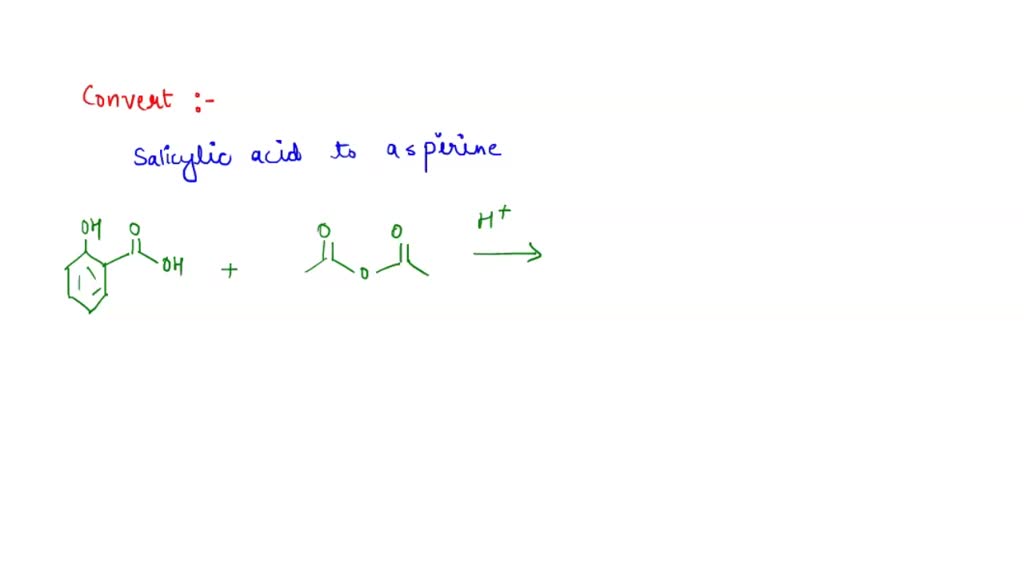
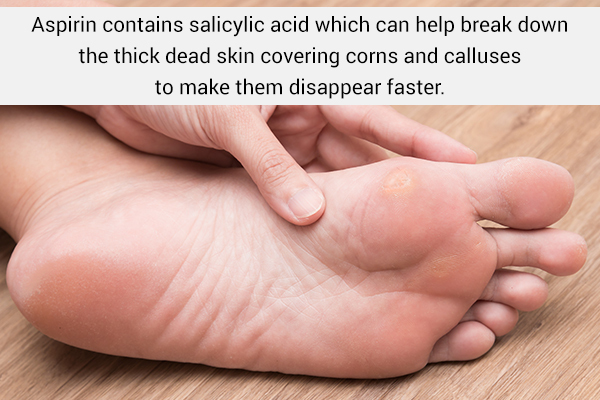
SOLVED: Carboxylic acid and phenol. Why would salicylic acid contain two acidic groups? Is it desirable to convert salicylic acid into aspirin (acetylsalicylic acid)?
![The active ingredient in aspirin is acetyl salicylic acid with\\[{{K}_{a}}=\\text{ }4.0\\times {{10}^{-9}}\\] . The pH of the solution obtained by dissolving two aspirin tablets (contain 0.36 g of acetyl salicylic acid in The active ingredient in aspirin is acetyl salicylic acid with\\[{{K}_{a}}=\\text{ }4.0\\times {{10}^{-9}}\\] . The pH of the solution obtained by dissolving two aspirin tablets (contain 0.36 g of acetyl salicylic acid in](https://www.vedantu.com/question-sets/214d4d7b-aa1e-4b43-8585-31cd4abd95614088496745223917951.png)
The active ingredient in aspirin is acetyl salicylic acid with\\[{{K}_{a}}=\\text{ }4.0\\times {{10}^{-9}}\\] . The pH of the solution obtained by dissolving two aspirin tablets (contain 0.36 g of acetyl salicylic acid in

Analysis of Decomposition Products in Aspirin by Application of Two-wavelength Detection : Hitachi High-Tech Corporation

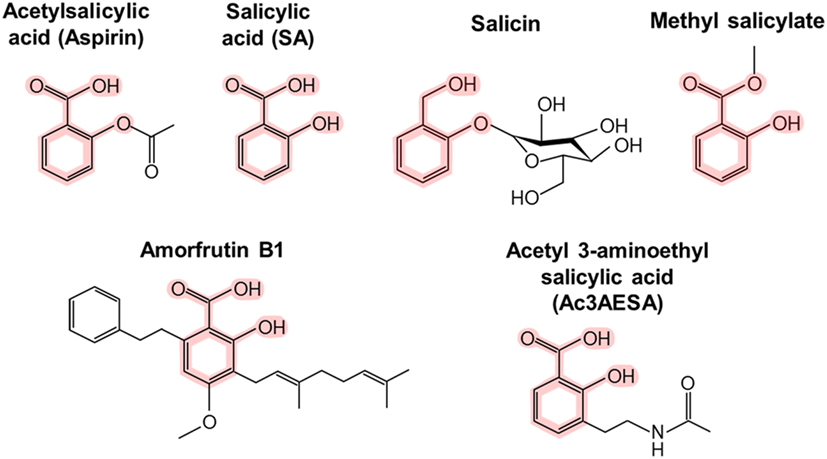
Chemical structures of salicylic acid and its synthetic and natural... | Download Scientific Diagram
When synthesizing aspirin in the lab, the product turns out to be a yellow solution and brown ppt when salicylic acid is reacted with acetic anhydride and H2SO4 (as a catalyst). What

.jpg_img_upload_solution_2022-07-16%2012:39:04.869270.png)



:max_bytes(150000):strip_icc()/GettyImages-1148718789-75fc732c872543908ddbac1d951686ec.jpg)