Foundation Medicine Introduces FoundationOne®Liquid, the Latest Advance in the Company's Liquid Biopsy Test for Solid Tumors in Patients with Advanced Cancer | Business Wire
Roche France, Foundation Medicine and the Institute Gustave Roussy announce unique partnership to provide in-house liquid biopsy genomic testing to cancer patients in France | Gustave Roussy

Foundation Medicine Eyes Liquid Biopsy Expansion, DNA Methylation Adoption, with Lexent Bio Acquisition | Inside Precision Medicine
92. Phil Stephens, Ph.D., CSO, Foundation Medicine- Session: Liquid Biopsy – Using the Right Tool for the Right Job on Vimeo

Foundation Medicine to launch liquid biopsy companion diagnostic following FDA approval - MedCity News
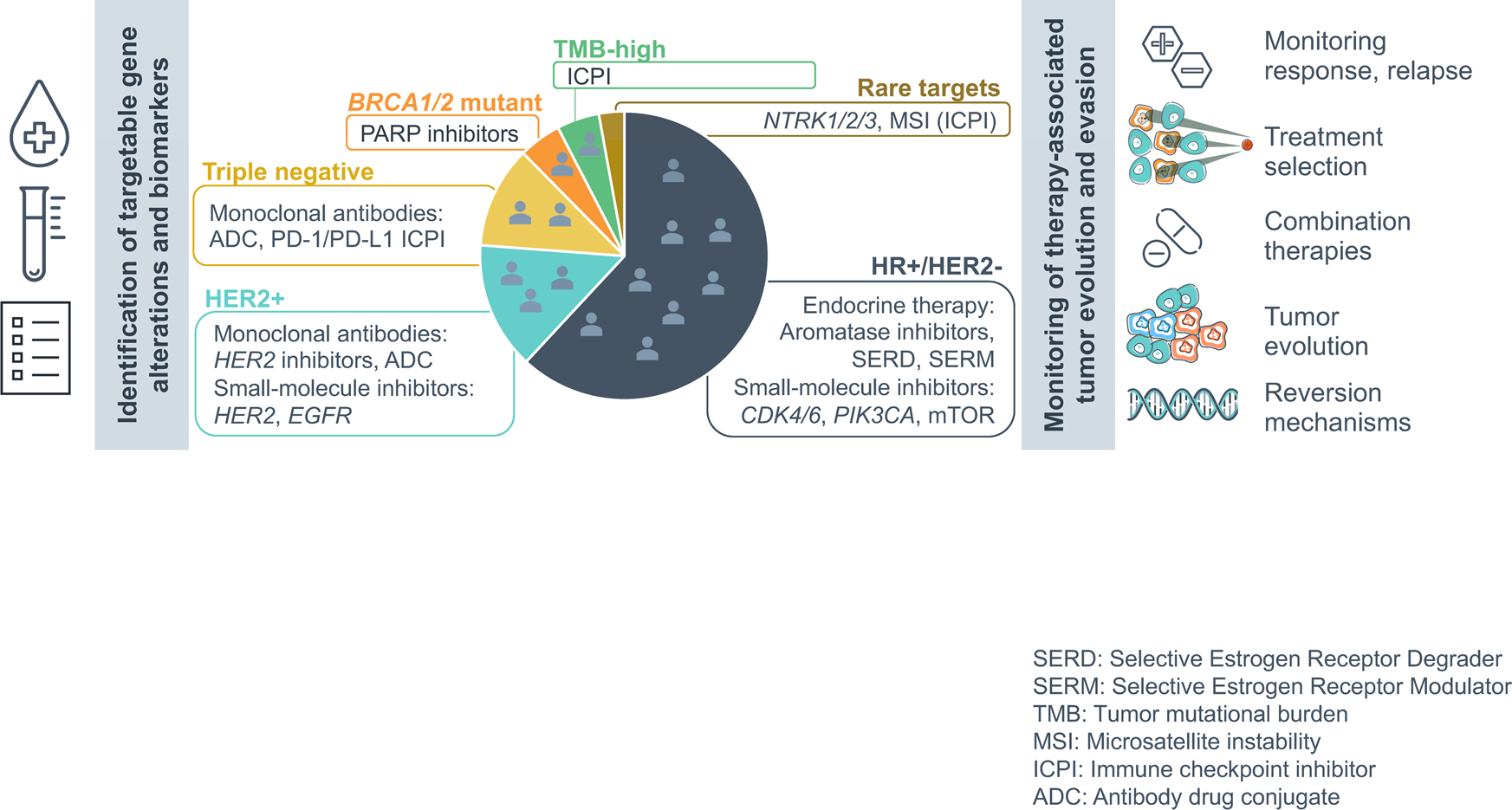
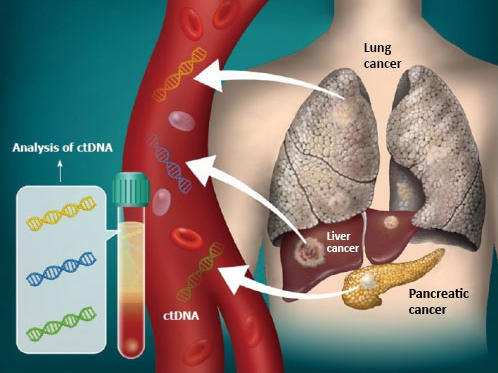

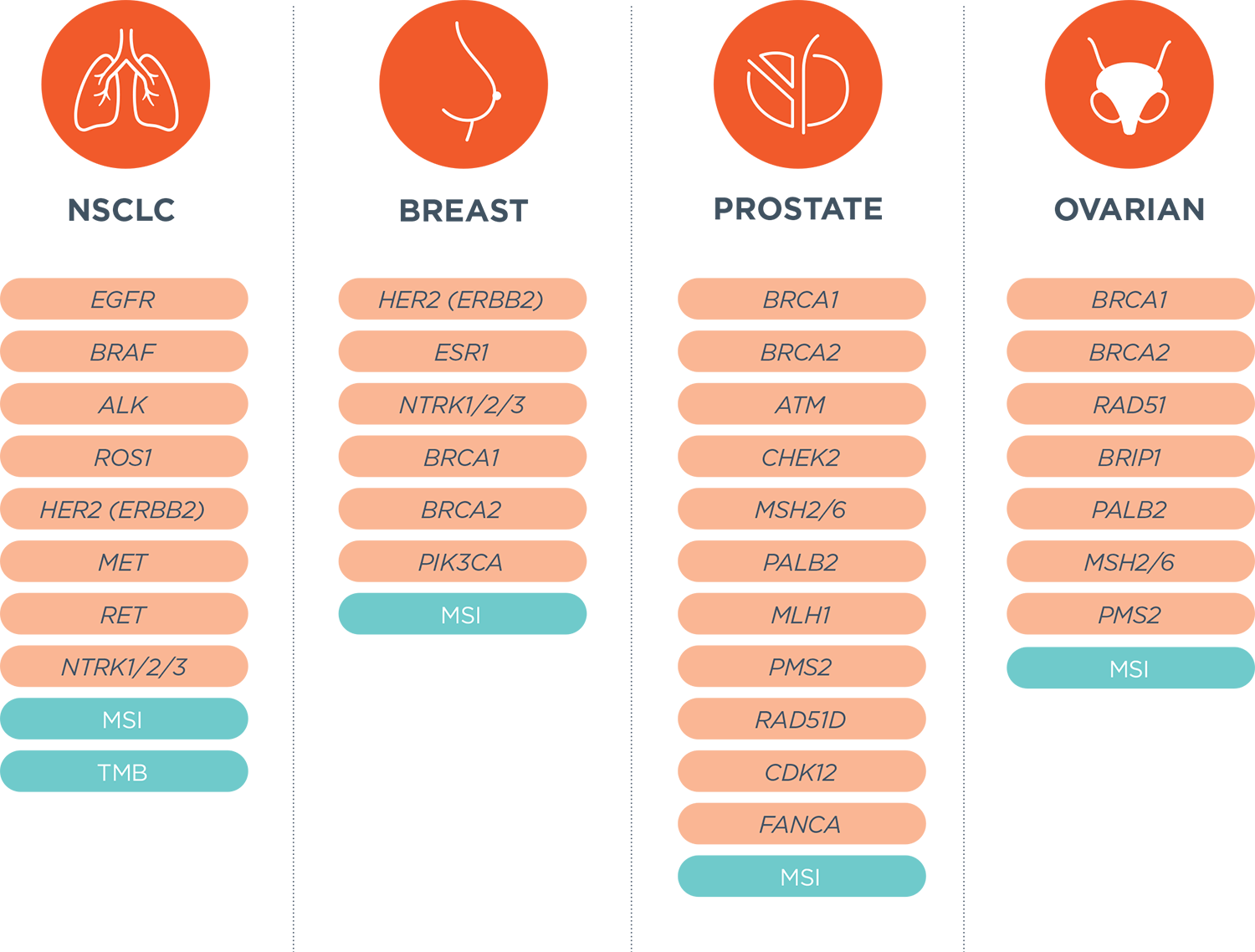
![Foundation Medicine review - 7 facts you should know [DEC 2021] Foundation Medicine review - 7 facts you should know [DEC 2021]](https://nebula.org/blog/wp-content/uploads/2021/12/FoundationOne-CDx-testing-kit.png)